RESEARCH PAPER
Antiretroviral therapy regimens associated with viral suppression in adolescents on HIV treatment in Kenya
1
National AIDS and STI Control Program (NASCOP), Kenya
2
Amref International University, Nairobi, Kenya
3
Technical University of Kenya, Nairobi, Kenya
Submission date: 2023-05-01
Final revision date: 2023-07-25
Acceptance date: 2023-07-25
Online publication date: 2025-11-20
HIV & AIDS Review 2025;24(4):274-280
KEYWORDS
TOPICS
ABSTRACT
Introduction:
According to 2021 UNAIDS report, there were approximately 99,159 adolescents living with human immunodeficiency virus (HIV) in Kenya, with a viral suppression rate of 67%. There are limited studies in Kenya on the types of regimens associated with viral suppression among adolescents. Therefore, this study aimed to determine the antiretroviral therapy (ART) regimens associated with viral load suppression in adolescents on ART.
Material and methods:
A retrospective cross-sectional analysis of 38,503 HIV-infected adolescents (age range, 10-19 years) receiving ART for at least 6 months with a documented viral load, was conducted. Data analyzed were Kenyan HIV program data obtained from electronic medical records of HIV-positive patients from January 2018 till December 2022.
Results:
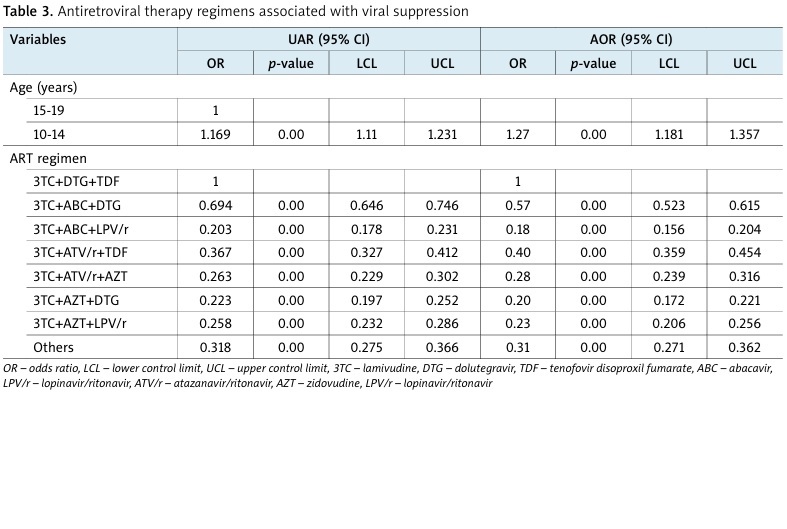
The viral suppression rate was 81.2%, distinctly higher than the 2021 UNAIDS estimate of 67% and the national suppression rate of 75% (2022). The highest viral suppression was found in the lamivudine (3TC) + dolutegravir (DTG) + tenofovir disoproxil fumarate (TDF) regimen (86.4%), followed by lamivudine (3TC) + abacavir (ABC) + dolutegravir (DTG) (81.6%) (AOR = 0.57, 95% CI: –0.52 to 1.65). Lamivudine (3TC) + atazanavir/ritonavir (ATV/r) + tenofovir (TDF) exhibited a viral suppression of 70.1% (AOR = 0.4, 95% CI: –0.34 to 0.45), lamivudine (3TC) + ritonavir/atazanavir (ATV/r) + zidovudine (AZT) had viral suppression of 62.7% (AOR = 0.28, 95% CI: –0.24 to 0.32), while lamivudine (3TC) + zidovudine (AZT)+ lopinavir/ritonavir (LPV/r) was found with 62.2% rate (AOR = 0.23, 95% CI: –0.21 to 0.26), lamivudine (3TC) + zidovudine (AZT) + dolutegravir (DTG) with 58.7% (AOR = 0.2, 95% CI: –0.17 to 0.22), and lamivudine (3TC) + abacavir (ABC) + lopinavir (LPV/r) with 56.4% (AOR = 0.18, 95% CI: –0.16 to 0.20).
Conclusions:
The viral suppression rate for adolescents was 81.2%. It is crucial to expedite treatment optimization for youths with non-optimal regimens to prevent treatment failure and ensure achieving the UNAIDS target of 95%. A comprehensive examination of viral suppression rates for adolescents on protease inhibitors is essential with consideration of shifting to optimal regimens, thereby improving treatment outcomes.
According to 2021 UNAIDS report, there were approximately 99,159 adolescents living with human immunodeficiency virus (HIV) in Kenya, with a viral suppression rate of 67%. There are limited studies in Kenya on the types of regimens associated with viral suppression among adolescents. Therefore, this study aimed to determine the antiretroviral therapy (ART) regimens associated with viral load suppression in adolescents on ART.
Material and methods:
A retrospective cross-sectional analysis of 38,503 HIV-infected adolescents (age range, 10-19 years) receiving ART for at least 6 months with a documented viral load, was conducted. Data analyzed were Kenyan HIV program data obtained from electronic medical records of HIV-positive patients from January 2018 till December 2022.
Results:
The viral suppression rate was 81.2%, distinctly higher than the 2021 UNAIDS estimate of 67% and the national suppression rate of 75% (2022). The highest viral suppression was found in the lamivudine (3TC) + dolutegravir (DTG) + tenofovir disoproxil fumarate (TDF) regimen (86.4%), followed by lamivudine (3TC) + abacavir (ABC) + dolutegravir (DTG) (81.6%) (AOR = 0.57, 95% CI: –0.52 to 1.65). Lamivudine (3TC) + atazanavir/ritonavir (ATV/r) + tenofovir (TDF) exhibited a viral suppression of 70.1% (AOR = 0.4, 95% CI: –0.34 to 0.45), lamivudine (3TC) + ritonavir/atazanavir (ATV/r) + zidovudine (AZT) had viral suppression of 62.7% (AOR = 0.28, 95% CI: –0.24 to 0.32), while lamivudine (3TC) + zidovudine (AZT)+ lopinavir/ritonavir (LPV/r) was found with 62.2% rate (AOR = 0.23, 95% CI: –0.21 to 0.26), lamivudine (3TC) + zidovudine (AZT) + dolutegravir (DTG) with 58.7% (AOR = 0.2, 95% CI: –0.17 to 0.22), and lamivudine (3TC) + abacavir (ABC) + lopinavir (LPV/r) with 56.4% (AOR = 0.18, 95% CI: –0.16 to 0.20).
Conclusions:
The viral suppression rate for adolescents was 81.2%. It is crucial to expedite treatment optimization for youths with non-optimal regimens to prevent treatment failure and ensure achieving the UNAIDS target of 95%. A comprehensive examination of viral suppression rates for adolescents on protease inhibitors is essential with consideration of shifting to optimal regimens, thereby improving treatment outcomes.
REFERENCES (18)
1.
UNAIDS. Global AIDS Update 2020 [Internet]. Geneva: UNAIDS; 2020. Available from: https://www.unaids.org/en/reso... (Accessed: 29.04.2023).
2.
UNICEF. Global Summary of HIV Epidemic among Adolescents aged 10-19, UNICEF Data: Monitoring the situation of children and women. [Internet]. New York: UNICEF; 2020. Available from: https://data.unicef.org/topic/... (Accessed: 29.04.2023).
3.
NASCOP. Kenya Population-based HIV Impact Assessment (KENPHIA) 2018 Preliminary Report [Internet]. Nairobi: Kenya Ministry of Health; 2020. Available from: https://www.health.go.ke/wp-co... (Accessed: 01.05.2023).
4.
Bispo S, Chaves L, Amaral M, de Sá CA. Type of antiretroviral regimen at initiation and its influence on viral load suppression: a retrospective cohort study in a Portuguese hospital. AIDS Res Hum Retroviruses 2021; 37: 383-388.
5.
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375: 830-839.
6.
Kenya National Bureau of Statistics. 2019 Kenya Population and Housing Census. Volume III: Distribution of Population by Age and Sex. December 2019. [Online]. Available from: https://www.knbs.or.ke/?wpdmpr...- volume-iii-distribution-of-population-by-age-sex-and-administrative- units.
7.
Su B, Wang Y, Zhou R, Jiang T, Zhang H, Li Z, et al. Efficacy and tolerability of lopinavir/ritonavir- and efavirenz-based initial antiretroviral therapy in HIV-1-infected patients in a Tertiary Care Hospital in Beijing, China. Front Pharmacol 2019; 10: 1472. DOI: 10.3389/fphar.2019.01472.
8.
Jacobson K, Ogbuagu O. Integrase inhibitor-based regimens result in more rapid virologic suppression rates among treatment-naïve human immunodeficiency virus-infected patients compared to non-nucleoside and protease inhibitor-based regimens in a real- world clinical setting: a retrospective cohort study. Medicine (Baltumore) 2018; 97: e13016. DOI: 10.1097/MD.0000000000013016.
9.
Taiwo BO, Marconi VC, Berzins B, Moser CB, Nyaku AN, Fichtenbaum CJ, et al. Dolutegravir plus lamivudine maintains human immunodeficiency virus-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis 2018; 66: 1868-1870.
10.
NAMSAL ANRS 12313 Study Group; Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, Eymard-Duvernay S, Leroy S, Boyer S, et al. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med 2019; 381: 816-826.
11.
Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al.; GEMINI Study Team. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393: 143-155.
12.
Dong BJ, Ward DJ, Chamberlain LA, Reddy YS, Ebrahimi R, Flaherty JF, Owen WF. Safety and effectiveness of tenofovir/emtricitabine or lamivudine plus ritonavir boosted atazanavir in treatment experienced HIV infected adults at two urban private medical practices. J Antivir Antiretrovir 2012; 4: 001-005. DOI: 10.4172/jaa.1000038.
13.
Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, et al. A phase III comparative study of the efficacy and tolerability of three non-nucleoside reverse transcriptase inhibitor-sparing anti retroviral regimens for treatment-naïve HIV-1-infected volunteers: a randomized, controlled trial. Ann Intern Med 2014; 161: 461. DOI: 10.7326/M14-1084.
14.
Kumar PN, Salvato P, LaMarca A, DeJesus E, Patel P, McClernon D, et al. A randomized, controlled trial of initial anti-retroviral therapy with abacavir/lamivudine/zidovudine twice-daily compared to atazanavir once-daily with lamivudine/zidovudine twice-daily in HIV-infected patients over 48 weeks (ESS100327, the ACTION Study). AIDS Res Ther 2009; 6: 3. DOI: 10.1186/1742-6405-6-3.
15.
Ndemwa P, Kilonzo J, Okinda N, Ng’ang’a L, Keter A, Maritim M, et al. Efficacy and safety of lopinavir/ritonavir-based regimens in HIV-infected patients in Kenya: a cohort study. PLoS One 2017; 12: e0173451. DOI: 10.1371/journal.pone.0173451.
16.
Gulick RM, Ribaudo HJ, Shikuma CM, Lalama C, Schackman BR, Meyer III WA, et al. Triple-nucleoside regimens versus efavirenz containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 2004; 350: 1850-1861.
17.
Kityo C, Sigaloff KC, Boender TS, Kaudha EN, Kayiwa JT, Dongho GB, et al. Virologic performance and durability of first-line antiretroviral therapy regimens in Uganda. J Acquir Immune Defic Syndr 2013; 62: 146-154.
18.
Musiime V, Keishanyu R, Kaudha E. Safety, pharmacokinetics and acceptability of the ABC/3TC/LPV/r GRANULES (4-in-1) in children living with HIV (3-20 kg) in Uganda: LOLIPOP study. Oral abstract 4. In: 12th International Workshop on HIV Paediatrics (virtual meeting); 2020. Available from: https://academicmedicaleducati....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.