RESEARCH PAPER
Impacts of HIV-1 and HIV-2 on the occurrence of opportunistic infections among HIV patients using HIV-1 anti-retroviral therapy in Njombe and Dar es Salaam, Tanzania: a retrospective cross sectional study
1
The Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
2
Ministry of Health – National TB and Leprosy Program, Tanzania
3
Kilimanjaro Clinical Research Institute, Kilimanjaro Christian Medical Centre, Moshi, Tanzania
4
Kilimanjaro Christian Medical University College, Moshi, Tanzania
5
Mbeya Medical Research Centre, National Institute of Medical Research, Mbeya, Tanzania
Submission date: 2023-06-09
Final revision date: 2024-04-29
Acceptance date: 2024-05-01
Online publication date: 2025-08-18
Corresponding author
Hamimu Omary Kigumi
Department of Health and Biomedical Sciences, School of Life Sciences and Biomedical Engineering, The Nelson Mandela African Institution of Sciences and Technology, Namabala, 255, Arusha, Tanzania
Department of Health and Biomedical Sciences, School of Life Sciences and Biomedical Engineering, The Nelson Mandela African Institution of Sciences and Technology, Namabala, 255, Arusha, Tanzania
HIV & AIDS Review 2025;24(4):267-273
KEYWORDS
TOPICS
ABSTRACT
Introduction:
HIV-1 and HIV-2 are globally known human immunodeficiency virus (HIV) types with 55% genetic difference. Partly dominated HIV-2 type is shown to spread to other countries due to immigration and socio-economic interactions. This study aimed to determine seroprevalence of HIV-1, HIV-2, and HIV-1/2 dual infection, and their impacts on the occurrence of opportunistic infections (OIs) among HIV-positive patients on antiretroviral therapy (ART) in Njombe and Dar es Salaam, Tanzania.
Material and methods:
A retrospective cross-sectional study was conducted at eight health facilities. A total of 300 participants were recruited. Patients’ history of OIs were obtained from patients’ files and interviews. SPSS version 26.0 were used for analysis. Ethical clearance was sought from KNCHRE. All participants provided signed informed consent.
Results:
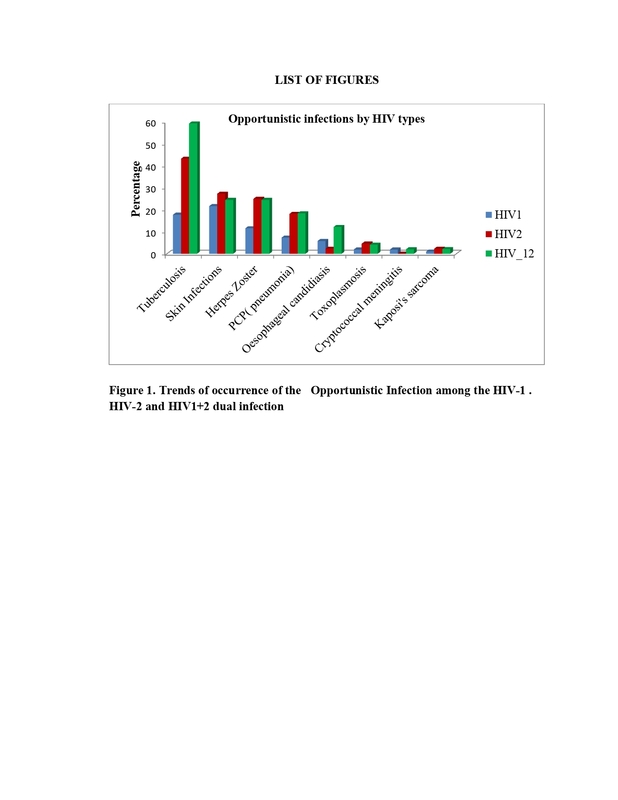
The mean age of participants was 35 (SD ± 0.24) years. The general prevalence of HIV-1 was 69%, HIV-2 was 15%, and HIV 1/2 dual infection was 16%. Tuberculosis, Pneumocystis pneumonia, and esophageal candidiasis were common OIs in HIV-1/2 dual infection type (p < 0.001, p = 0.02, p = 0.02, respectively). HIV-2 and HIV-1/2 had two times higher risks of OIs (RR: 1.69, 95% CI: 1.39-2.07%, p < 0.001; and RR: 1.78, 95% CI: 1.51-2.10%, p < 0.001, respectively).
Conclusions:
The study confirmed the presence of HIV-2 and HIV-1/2 dual infection in Tanzania. Additionally, it showed high-risk of OIs occurrence among HIV-2 and HIV-1/2 people living with HIV (PLHIV). Therefore, the initiation of HIV-2 ART regimen in Tanzania should be established to reduce poor treatment outcomes among HIV-2 and HIV-1/2 PLHIV.
HIV-1 and HIV-2 are globally known human immunodeficiency virus (HIV) types with 55% genetic difference. Partly dominated HIV-2 type is shown to spread to other countries due to immigration and socio-economic interactions. This study aimed to determine seroprevalence of HIV-1, HIV-2, and HIV-1/2 dual infection, and their impacts on the occurrence of opportunistic infections (OIs) among HIV-positive patients on antiretroviral therapy (ART) in Njombe and Dar es Salaam, Tanzania.
Material and methods:
A retrospective cross-sectional study was conducted at eight health facilities. A total of 300 participants were recruited. Patients’ history of OIs were obtained from patients’ files and interviews. SPSS version 26.0 were used for analysis. Ethical clearance was sought from KNCHRE. All participants provided signed informed consent.
Results:
The mean age of participants was 35 (SD ± 0.24) years. The general prevalence of HIV-1 was 69%, HIV-2 was 15%, and HIV 1/2 dual infection was 16%. Tuberculosis, Pneumocystis pneumonia, and esophageal candidiasis were common OIs in HIV-1/2 dual infection type (p < 0.001, p = 0.02, p = 0.02, respectively). HIV-2 and HIV-1/2 had two times higher risks of OIs (RR: 1.69, 95% CI: 1.39-2.07%, p < 0.001; and RR: 1.78, 95% CI: 1.51-2.10%, p < 0.001, respectively).
Conclusions:
The study confirmed the presence of HIV-2 and HIV-1/2 dual infection in Tanzania. Additionally, it showed high-risk of OIs occurrence among HIV-2 and HIV-1/2 people living with HIV (PLHIV). Therefore, the initiation of HIV-2 ART regimen in Tanzania should be established to reduce poor treatment outcomes among HIV-2 and HIV-1/2 PLHIV.
REFERENCES (28)
2.
Tanzania National AIDS Control Program (NACP) report. 2012. Available at: https://nacp.go.tz/reports/.
3.
Hamelar J. The original and diversity of the HIV-1 pandemic. Trends Mol Med 2012; 18: 182-192.
4.
Bobkov AF, Kazennova EV, Selimova LM, Khanina TA, Ryabov GS, Bobkova MR, et al. Temporal trends in the HIV-1 epidemic in Russia: predominance of subtype A. J Med Virol 2004; 74: 191-196.
5.
Kinney RG, Spach DH. Comparison of HIV-1 AND HIV-2 among the HIV key population. https://www.hiv.uw.edu/go/key-....
6.
Shao ER, Kifaro EG, Kimaro J, Mrema JG, Mwasamwaja AO, Kayandabila J, Nyombi BM. HIV-1 diversity in Tanzania and its implication toward development of effective vaccines: a review article. Journal of Vaccines & Vaccination 2014; 5: 5. DOI: 10.4172/21577560.1000249.
7.
Centers for Disease Control (CDC). Global update of epidemiology of HIV infection. Origin of the HIV epidemic. 2019. Available at: https://www.cdc.gov/hiv-data/n...- prevalence.html.
8.
Gottlieb GS, Eholié SP, Nkengasong J, Jallow S, Rowland-Jones S, Whittle HC, Sow PS. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS 2008; 22: 2069-2072.
9.
Esbjörnsson J, Jansson M, Jespersen S, Månsson F, Hønge BL, Lindman J, et al. HIV-2 as a model to identify a functional HIV cure. AIDS Res Ther 2019; 16: 24. DOI: 10.1186/s12981-019-0239-x.
10.
Peterson K, Jallow S, Rowland-Jones SL, de Silva TI. Antiretroviral therapy for HIV-2 infection: recommendations for management in low-resource settings. AIDS Res Treat 2011; 2011: 463704. DOI: 10.1155/2011/463704.
11.
Jallow S, Alabi A, Sarge-Njie R, Peterson K, Whittle H, Corrah T, et al. Virological response to highly active antiretroviral therapy in patients infected with human immunodeficiency virus type 2 (HIV-2) and in patients dually infected with HIV-1 and HIV-2 in the Gambia and emergence of drug-resistant variants. J Clin Microbiol 2009; 47: 2200-2208.
12.
National Bureau of Statistics Tanzania. A preliminary survey for existence of HIV1, HIV2 co-infection and AIDS dementia in Tanzania; 2019. Available at: https://www.nbs.go.tz/uploads/....
13.
Plantier JC, Gueudin M, de Oliveira F, Damond F, Lemée V, Brun- Vézinet F, Simon F. Rapid discrimination between human immunodeficiency virus type 2 groups A and B by real-time PCR. J Clin Microbiol 2004; 42: 5866-5870.
14.
Heredia A, Vallejo A, Soriano V, Aguilera A, Mas A, Epstein JS, Hewlett IK. Genetic analysis of an HIV type 2 subtype B virus from a Spanish individual with AIDS. AIDS Res Hum Retroviruses 1997; 13: 899-900.
15.
Visseaux B, Damond F, Matheron S, Descamps D, Charpentier C. Corrigendum to "HIV-2 molecular epidemiology" [Infect. Genet. Evol. 46 (2016) 233-240]. Infect Genet Evol 2018; 58: 294. DOI: 10.1016/j.meegid.2017.12.024.
16.
The United Republic of Tanzania. Ministry of Health, Community Development, Gender, Elderly, and Children. National AIDS Control Programme. National Guidelines for the Management of HIV and AIDS. 2019. Available at: https://www.differentiatedserv....
17.
Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, et al.; ANRS 12222 Morbidity/Mortality Study Group. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d'Ivoire. Clin Infect Dis 2012; 54: 714-723.
18.
Selwyn PA, Pumerantz AS, Durante A, Alcabes PG, Gourevitch MN, Boiselle PM, Elmore JG. Clinical predictors of Pneumocystis carinii pneumonia, bacterial pneumonia and tuberculosis in HIV- infected patients. AIDS 1998; 12: 885-893.
19.
Fish DG, Ampel NM, Galgiani JN, Dols CL, Kelly PC, Johnson CH, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 1990; 69: 384-391.
20.
van der Sande MA, Schim van der Loeff MF, Bennett RC, Dowling M, Aveika AA, Togun TO, et al. Incidence of tuberculosis and survival after its diagnosis in patients infected with HIV-1 and HIV-2. AIDS 2004; 18: 1933-1941.
21.
Nyamogoba HD, Mbuthia G, Mining S, Kikuvi G, Biegon R, Mpoke S, et al. HIV co-infection with tuberculous and non-tuberculous mycobacteria in western Kenya: challenges in the diagnosis and management. Afr Health Sci 2012; 12: 305-311.
22.
Gustafson P, Gomes VF, Naucler A, Vieira CS, Samb B, Aaby P, et al. Clinical predictors for death in HIV-positive and HIV negative tuberculosis patients in Guinea-Bissau. Infection 2007; 35: 69-80.
23.
Ghate M, Deshpande S, Tripathy S, Nene M, Gedam P, Godbole S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: analysis by stages of immunosuppression represented by CD4 counts. J Infect Dis 2009; 13: e1-e8. DOI: 10.1016/j.ijid.2008.03.029.
24.
The United Republic of Tanzania. National Bureau of Statistics. Ministry of Finance. Tanzania in Figures 2012. Available at: https://www.nbs.go.tz/nbs/takw....
25.
Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC). Tanzania HIV Impact Survey (THIS) 2016-2017: Final Report. Dar es Salaam, Tanzania. December 2018. Available at: https://www.nbs.go.tz/nbs/takw....
26.
Harries K, Zachariah R, Manzi M, Firmenich P, Mathela R, Drabo J, et al. Baseline characteristics, response to and outcome of antiretro viral therapy among patients with HIV-1, HIV-2 and dual infection in Burkina Faso. Trans R Soc Trop Med Hyg 2010; 104: 154-161.
27.
Smith CJ. Toward Optimal ART for HIV-2 Infection: Can Genotypic and Phenotypic Drug Resistance Testing Help Guide Therapy in HIV-2? Paper # 579. San-Francisco, USA: 17th Conference on Retoviruses and Opportunistic Infections; 2010.
28.
Prince PD, Matser A, van Tienen C, Whittle HC, Schim van der Loeff MF. Mortality rates in people dually infected with HIV-1/2 and those infected with either HIV-1 or HIV-2: a systematic review and meta-analysis. AIDS 2014; 28: 549-558.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.