RESEARCH PAPER
Prevalence and associated factors of insulin resistance among HIV-infected patients receiving antiretroviral therapy:
a cross-sectional study from Tunisia
1
University of Monastir, Tunisia
2
Department of Infectious Disease, Fattouma Bourguiba University Hospital, Monastir, Tunisia
3
Department of Endocrinology, Fattouma Bourguiba University Hospital, Monastir, Tunisia
4
Laboratory of Biochemistry-Toxicology, Fattouma Bourguiba University Hospital, Monastir, Tunisia
Submission date: 2022-01-25
Final revision date: 2022-05-08
Acceptance date: 2022-06-08
Publication date: 2025-09-07
Corresponding author
Ikbel Kooli
University of Monastir, Service de Maladies Infectieuses CHU Fattouma Bour, 5000, Monastir, Tunisia
University of Monastir, Service de Maladies Infectieuses CHU Fattouma Bour, 5000, Monastir, Tunisia
HIV & AIDS Review 2025;24(3):195-200
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Antiretroviral therapy (ART) has significantly improved prognosis of human immunodeficiency virus (HIV) infection by reducing both morbidity and mortality rates. However, this therapy leads to an increased incidence of metabolic disorders, such as insulin resistance (IR). The aim of this study was to determine the prevalence of IR in non-diabetic HIV-infected patients receiving ART, and to investigate the potentially associated factors.
Material and methods:
We conducted a cross-sectional study among 67 non-diabetic HIV-infected patients receiving ART. IR was determined through homeostasis model assessment (HOMA-IR). Bivariate and multivariate analyses were performed to investigate the association between demographic, clinical, and biological variables, and IR.
Results:
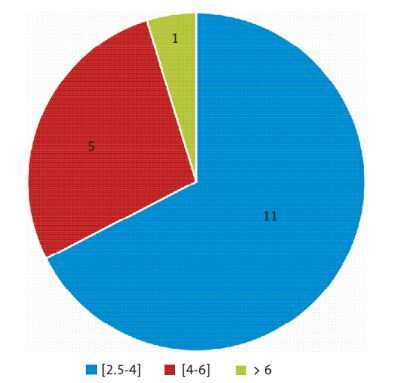
A total of 67 HIV-infected patients were enrolled, among whom 43 (64.2%) were males, and the median age was 38.7 years. The prevalence of metabolic syndrome was 28.3%, and IR was found in 30 patients (44.7%). Seventeen of these patients had not a metabolic syndrome. HOMA-IR values were ranging between 2 and 3.99 in 11 patients (64.7%), between 4 and 6 in 5 patients (29.4%), and was greater than 6 in one patient. In the multivariate analysis, there was no significant association between body mass index, CD4+ count, ART regimen, and IR.
Conclusions:
Asymptomatic IR, responsible in the long-term for the occurrence of other serious metabolic disorders, is common among HIV-infected patients, and cannot be predictable. Screening of insulin resistance by measuring HOMA-IR is the main parameter for early detection of metabolic risk and personalized management.
Antiretroviral therapy (ART) has significantly improved prognosis of human immunodeficiency virus (HIV) infection by reducing both morbidity and mortality rates. However, this therapy leads to an increased incidence of metabolic disorders, such as insulin resistance (IR). The aim of this study was to determine the prevalence of IR in non-diabetic HIV-infected patients receiving ART, and to investigate the potentially associated factors.
Material and methods:
We conducted a cross-sectional study among 67 non-diabetic HIV-infected patients receiving ART. IR was determined through homeostasis model assessment (HOMA-IR). Bivariate and multivariate analyses were performed to investigate the association between demographic, clinical, and biological variables, and IR.
Results:
A total of 67 HIV-infected patients were enrolled, among whom 43 (64.2%) were males, and the median age was 38.7 years. The prevalence of metabolic syndrome was 28.3%, and IR was found in 30 patients (44.7%). Seventeen of these patients had not a metabolic syndrome. HOMA-IR values were ranging between 2 and 3.99 in 11 patients (64.7%), between 4 and 6 in 5 patients (29.4%), and was greater than 6 in one patient. In the multivariate analysis, there was no significant association between body mass index, CD4+ count, ART regimen, and IR.
Conclusions:
Asymptomatic IR, responsible in the long-term for the occurrence of other serious metabolic disorders, is common among HIV-infected patients, and cannot be predictable. Screening of insulin resistance by measuring HOMA-IR is the main parameter for early detection of metabolic risk and personalized management.
REFERENCES (30)
1.
UNAIDS. Global HIV & AIDS statistics – 2020 fact sheet. Available at: https://www.unaids.org/en/reso... (Accessed: 10.05.2021).
2.
Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord, Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol 2012; 41: 433 445.
3.
Hanttu A, Kauppinen KJ, Kivelä P, Ollgren J, Jousilahti P, Liitsola K, et al. Prevalence of obesity and disturbances in glucose homeostasis in HIV-infected subjects and general population-missed diagnoses of diabetes? HIV Med 2021; 22: 244-253.
4.
Safro FS, Norman B, Nichols M, Appiah L, Osei Assibey S, Tagge R, et al. Prevalence and incidence of pre-diabetes and diabetes mellitus among people living with HIV in Ghana: evidence from the EVERLAST study. HIV Med 2021; 22: 231-243.
5.
Pedro MN, Rocha GZ, Guadagnini D, Santos A, Magro DO, Assa-lin HB, et al. Insulin resistance in HIV-patients: causes and consequences. Front Endocrinol (Lausanne) 2018; 9: 514. DOI: 10.3389/fendo.2018.00514.
6.
Robert JJ. Methods for the measurement of insulin resistance. Hyperinsulinemic euglycemic clamp. Presse Med 1995; 24: 730-734.
7.
Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Salmon-Ceron D, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012; 26: 303 314.
8.
Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 2009; 50: 499 505.
9.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214-E223. DOI: 10.1152/ajpendo.1979.237.3.E214.
10.
Otten J, Ahrén B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic-euglycaemic clamp: a meta-analysis. Diabetologia 2014; 57: 1781 1788.
11.
Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000; 23: 57 63.
12.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85: 2402 2410.
13.
Tang Q, Li X, Song P, Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: developments in research and prospects for the future. Drug Discov Ther 2015; 9: 380 385.
14.
Marques-Vidal P, Mazoyer E, Bongard V, Gourdy P, Puidavets JB, Drouet L, et al. Prevalence of insulinresistance syndrome in Southwestern France and its relationship with inflammatory and homeostatic makers. Diabetes Care 2002; 25: 1371-1377.
15.
Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013; 13: 47. DOI: 10.1186/1472-6823-13-47.
16.
Timoteo AT, Miranda F, Carmo MM, Ferreira RC. Optimal cut-off value for homeostasis model assessment (HOMA) index of insulin-resistance in a population of patients admitted electively in a Portuguese cardiology ward. Acta Med Port 2014; 27: 473-479.
17.
Araujo S, Banon S, Machuca I, Moreno A, Perez-Elias MJ, Casado JL. Prevalence of insulin resistance and risk of diabetes mellitus in HIV-infected patients receiving current antiretroviral drugs. Eur J Endocrinol 2014; 171: 545-554.
18.
Schulte-Hermann K, Schalk H, Haider B, Hutterer J, Gmeinhart B, Pichler K, et al. Impaired lipid profile and insulin resistance in a cohort of austrian HIV patients. J Infect Chemother 2016; 22: 248-253.
19.
De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new-onset diabetes in HIV-Infected Patients. The data collection on adverse events of anti-HIV drugs (D:A:D) study. Diabetes Care 2008; 31: 1224 1229.
20.
Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care 2007; 30: 113 119.
21.
Noumegni SRN, Nansseu JR, Ama VJM, Bigna JJ, Assah FK, Guewo-Fokeng M, et al. Insulin resistance and associated factors among HIV-infected patients in sub-Saharan Africa: a cross sectional study from Cameroon. Lipids Health Dis 2017; 16: 148. DOI: 10.1186/s12944-017-0543-1.
22.
Feeney ER, Mallon PW. Insulin resistance in HIV infection. Best Pract Res Clin Endocrinol Metab 2011; 25: 443-458.
23.
Hulgan T. Factors associated with insulin resistance in adults with HIV receiving contemporary antiretroviral therapy: a brief update. Curr HIV AIDS Rep 2018; 15: 223-232.
24.
Oufassa F, Goujard C, Viard JP, Carlier R, Lefebvre B, Yeni P, et al. Immune deficiency could be an early risk factor for altered insulin sensitivity in antiretroviral-naive HIV-1-infected patients: the ANRS COPANA cohort. Antivir Ther 2012; 17: 91 100.
25.
El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med 2005; 6: 114 121.
26.
Limonel P, Biglino A, Valle M, Degioanni M, Paola Servato M, Berardi C, et al. Insulin reistance in HIV-infected patients: relationship with pro-inflammatory cytokines released by peripheral leukocytes. J Inf Secur 2003; 47: 52-58.
27.
Jadhav N, Bang A. A cross sectional study to determine prevalence of insulin resistance among HIV positive subjects on antiretroviral therapy. Int J Cont Med Res 2019; 6: 43-46.
28.
Walli R, Herfort O, Michl GM, Demant T, Jäger H, Dieterle C, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS 1998; 12: F167-F173.
29.
Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem 2000; 275: 20251-20254.
30.
Non LR, Escota GV, Powderly WG. HIV and its relationship to insulin resistance and lipid abnormalities. Transl Res 2017; 183: 41-56.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.